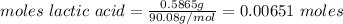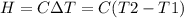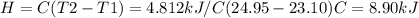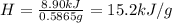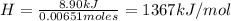Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 05:30
What royal scientist used the 29th day of frozen vapor to encounter elements for mastering new culinary creations?
Answers: 1

Chemistry, 22.06.2019 05:30
The climate of the continental united states is generally 1. tropical 2. temperate 3. arctic 4. highland
Answers: 1

Chemistry, 22.06.2019 08:30
What is the independent variable in this investigation? mass volume sample number substance density
Answers: 3
You know the right answer?
A0.5865-g sample of lactic acid (hc3h5o3) is burned in a calorimeter whose heat capacity is 4.812 kj...
Questions


Mathematics, 03.02.2021 19:20

Mathematics, 03.02.2021 19:20

Business, 03.02.2021 19:20


Social Studies, 03.02.2021 19:20


Mathematics, 03.02.2021 19:20

History, 03.02.2021 19:20


Advanced Placement (AP), 03.02.2021 19:20

Mathematics, 03.02.2021 19:20



Mathematics, 03.02.2021 19:20

History, 03.02.2021 19:20

Mathematics, 03.02.2021 19:20

History, 03.02.2021 19:20

Mathematics, 03.02.2021 19:20

Social Studies, 03.02.2021 19:20