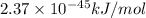Chemistry, 05.09.2019 18:30 ghwolf4p0m7x0
In solid nacl, the equilibrium separation between neighboring na+ and cl- ions is 0.283 nm. calculate the coulombic energy between na+ and at this distance. give your answer in each of j, ev, and kj/mol units.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:30
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2

Chemistry, 22.06.2019 19:00
What is the compound name for the formula [ru(en)2cl2]2+ and [co(en)cl2br]-
Answers: 1

Chemistry, 22.06.2019 20:20
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3

You know the right answer?
In solid nacl, the equilibrium separation between neighboring na+ and cl- ions is 0.283 nm. calculat...
Questions

Law, 22.01.2021 02:20

History, 22.01.2021 02:20



Mathematics, 22.01.2021 02:20


Mathematics, 22.01.2021 02:20



Advanced Placement (AP), 22.01.2021 02:20



Health, 22.01.2021 02:20

Mathematics, 22.01.2021 02:20


History, 22.01.2021 02:20

Mathematics, 22.01.2021 02:20


Mathematics, 22.01.2021 02:20

Mathematics, 22.01.2021 02:20

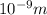 .
.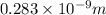
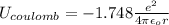
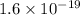 C
C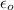 =
= 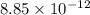
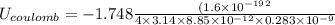
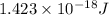
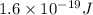
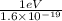
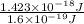
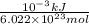
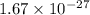 kJ/mol
kJ/mol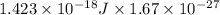 kJ/mol
kJ/mol