Chemistry, 05.09.2019 18:30 itzdryoshi
Nitric acid is often manufactured from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming of natural gas, in a two-step process. in the first step, nitrogen and hydrogen react to form ammonia: n2(g) + 3h2(g) > 2nh3(g) in the second step, ammonia and oxygen react to form nitric acid and water: nh3(g) + 2o2(g) > hno3(g) + h2o(g) write the net chemical equation for the production of nitric acid from nitrogen, hydrogen and oxygen. be sure your equation is balanced.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1

Chemistry, 22.06.2019 22:30
Draw the aromatic compound toluene (methylbenzene). show all hydrogen atoms, including those on the ring.
Answers: 1

Chemistry, 23.06.2019 01:00
Which of the following is in the lanthanide family? a) uranium b) promethium c) silver d) gold
Answers: 2
You know the right answer?
Nitric acid is often manufactured from the atmospheric gases nitrogen and oxygen, plus hydrogen prep...
Questions




Physics, 29.06.2021 21:40


Mathematics, 29.06.2021 21:50

Mathematics, 29.06.2021 21:50

Business, 29.06.2021 21:50



Mathematics, 29.06.2021 21:50

Business, 29.06.2021 21:50

Mathematics, 29.06.2021 21:50


Biology, 29.06.2021 21:50


Mathematics, 29.06.2021 21:50


Mathematics, 29.06.2021 21:50

English, 29.06.2021 21:50

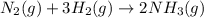 ......(1)
......(1)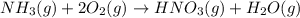 .....(2)
.....(2)