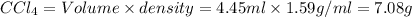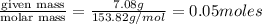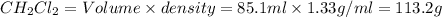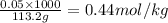Chemistry, 05.09.2019 17:30 kayelynn003
What is the molality of a solution consisting of 4.45 ml of carbon tetrachloride (ccl4; d = 1.59 g/ml) in 85.1 ml of methylene chloride (ch2cl2; d = 1.33 g/ml)?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which solution is an example of a nonelectrolyte? a. iodine in hexane b. sodium nitrate in waterc. acetic acid in waterd. hydrogen chloride in water
Answers: 2

Chemistry, 22.06.2019 00:30
In numbering carbon atoms in the parent chain of a hydrocarbon, why would you number from right to left, rather than left to right
Answers: 1

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2

Chemistry, 22.06.2019 20:30
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1
You know the right answer?
What is the molality of a solution consisting of 4.45 ml of carbon tetrachloride (ccl4; d = 1.59 g/...
Questions


Mathematics, 10.10.2019 21:40

History, 10.10.2019 21:40

Mathematics, 10.10.2019 21:40

Mathematics, 10.10.2019 21:40

Mathematics, 10.10.2019 21:40








History, 10.10.2019 21:40


Computers and Technology, 10.10.2019 21:40

Advanced Placement (AP), 10.10.2019 21:40



Mathematics, 10.10.2019 21:40

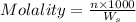
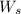 = weight of solvent in g
= weight of solvent in g