
Chemistry, 05.09.2019 17:30 bairdmatthew43
Solute s has a partition coefficient of 6.2 between water (phase 1) and hexane (phase 2). 74.0 ml solution of s in water is extracted six times with 17.0 ml of hexane. calculate the fraction of s remaining in the aqueous phase.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 11:00
Surface currents are caused by blank space . question 14 options: surface currents are caused by? differences in water temperature high salinity differences in density wind forces
Answers: 1

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2

Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1
You know the right answer?
Solute s has a partition coefficient of 6.2 between water (phase 1) and hexane (phase 2). 74.0 ml so...
Questions

Mathematics, 19.03.2021 21:10

Mathematics, 19.03.2021 21:10

Mathematics, 19.03.2021 21:10

Mathematics, 19.03.2021 21:10

Mathematics, 19.03.2021 21:10

Mathematics, 19.03.2021 21:10

Mathematics, 19.03.2021 21:10


Biology, 19.03.2021 21:10


Mathematics, 19.03.2021 21:10

History, 19.03.2021 21:10

History, 19.03.2021 21:10

History, 19.03.2021 21:10

Mathematics, 19.03.2021 21:10

Social Studies, 19.03.2021 21:10


Social Studies, 19.03.2021 21:10

Mathematics, 19.03.2021 21:10

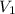 = 74.0 mL
= 74.0 mL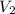 = 17.0 mL
= 17.0 mL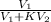
![[\frac{V_{1}}{V_{1} + KV_{2}}]^{3}](/tpl/images/0223/5422/324f2.png)
![[\frac{74.0 ml}{74.0 ml + 6.2 \times 17.0 ml}]^{3}](/tpl/images/0223/5422/2c23d.png)
![[\frac{74.0 ml}{179.4 ml}]^{3}](/tpl/images/0223/5422/f6b3c.png)


