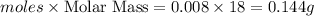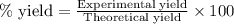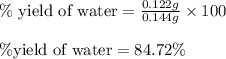Chemistry, 05.09.2019 16:20 kaymillsaps
Gaseous methane (ch4) reacts with gaseous oxygen gas (o2) to produce gaseous carbon dioxide (co2) and gaseous water (h2o) . if 0.122g of water is produced from the reaction of 0.16g of methane and 0.25g of oxygen gas, calculate the percent yield of water.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Acamcorder has a power rating of 17 watts. if the output voltage from its battery is 7 volts, what current does it use?units:
Answers: 1

Chemistry, 22.06.2019 11:30
If we compare and contrast electromagnetic waves with sound waves, all but one statement is true. that is a) sound waves require a medium to travel while electromagnetic waves do not. b) electromagnetic waves can travel through the vacuum of space while sound waves cannot. c) electromagnetic waves must have a medium in which to travel, but sound waves can travel anywhere. eliminate d) sound waves must bounce off of matter in order to travel while electromagnetic waves do not require matter to be present.
Answers: 3

Chemistry, 22.06.2019 15:00
Why does a plastic bottle that is sealed at a high altitude change it’s shape when taken to lower altitude
Answers: 2

Chemistry, 23.06.2019 03:20
What kind of intermolecular forces act between a hydrogen fluoride molecule and a hydrogen peroxide molecule? note: if there is more than one type of intermolecular force that acts, be sure to list them all, with a comma between the name of each force.
Answers: 1
You know the right answer?
Gaseous methane (ch4) reacts with gaseous oxygen gas (o2) to produce gaseous carbon dioxide (co2) an...
Questions




Mathematics, 02.12.2021 03:00



Computers and Technology, 02.12.2021 03:00

Mathematics, 02.12.2021 03:00

Mathematics, 02.12.2021 03:00

Mathematics, 02.12.2021 03:00




SAT, 02.12.2021 03:00


Mathematics, 02.12.2021 03:10



Computers and Technology, 02.12.2021 03:10

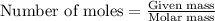 .....(1)
.....(1)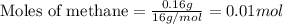
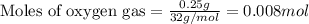
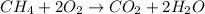
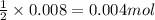 of methane
of methane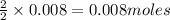 of water
of water