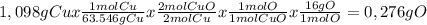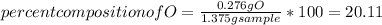Chemistry, 05.09.2019 16:10 alyssatamayo641
When 1.375 g of copper (ii) oxide is reduced on heating in a current of hydrogen, the weight of copper remaining after the reaction is complete is 1.098 g. what is the percent composition of oxygen?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
The best solution for preventing harm to people and pets from severe hurricanes involves determining and warning residents about what
Answers: 1

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 11:00
Iron (3) oxide will decompose in the presence of hydrogen gas and heater to produced iron and digydrogen monoxide white a balanced chemical equation
Answers: 1

Chemistry, 22.06.2019 19:50
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3
You know the right answer?
When 1.375 g of copper (ii) oxide is reduced on heating in a current of hydrogen, the weight of copp...
Questions

History, 11.07.2019 04:40



English, 11.07.2019 04:40



History, 11.07.2019 04:40


Mathematics, 11.07.2019 04:40

Mathematics, 11.07.2019 04:40

Mathematics, 11.07.2019 04:40

Biology, 11.07.2019 04:40

Chemistry, 11.07.2019 04:40

Business, 11.07.2019 04:40

Business, 11.07.2019 04:40

Mathematics, 11.07.2019 04:40


Mathematics, 11.07.2019 04:40

Social Studies, 11.07.2019 04:40

English, 11.07.2019 04:40