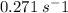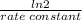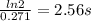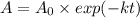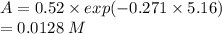Chemistry, 05.09.2019 04:10 mollykay2001p3qo0j
Molecular iodine, i2(g), dissociates into iodine atoms at 625 k with a first-order rate constant of 0.271 s−1.
(a) what is the half-life for this reaction?
(b) if you start with 0.052 m i2 at this temperature, how much will remain after 5.16 s assuming that the iodine atoms do not recombine to form i2?

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 14:30
Ahypothesis must be testable and falsifiable to be considered scientific a. trueb. false
Answers: 1

Chemistry, 22.06.2019 21:50
Given the data below for the reaction, 2 a + 2 b + 4 c => d + e + 3 f, the reaction is fill in the [ ] order in a, fill in the [ ] order in b, fill in the [ ] order in c and fill in the [ ] order overall. (use the words "first, second, third, fourth" to fill each blank)experimentinitial conc of a, mol/l initial conc of b, mol/l initial conc of c, mol/l initial rate, mol/l.s1 0.1 0.1 0.2 2 x 10-32 0.2 0.3 0.2 6 x 10-33 0.3 0.1 0.2 2 x 10-34 0.4 0.3 0.4 1.2 x 10-2
Answers: 2
You know the right answer?
Molecular iodine, i2(g), dissociates into iodine atoms at 625 k with a first-order rate constant of...
Questions

Mathematics, 14.05.2021 18:50



Social Studies, 14.05.2021 18:50


Mathematics, 14.05.2021 18:50


Computers and Technology, 14.05.2021 18:50

History, 14.05.2021 18:50

Biology, 14.05.2021 18:50


Mathematics, 14.05.2021 18:50

Geography, 14.05.2021 18:50






Mathematics, 14.05.2021 18:50