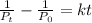Hi(g) decomposes to molecular hydrogen and molecular iodine by a
second-order reaction. if the...

Hi(g) decomposes to molecular hydrogen and molecular iodine by a
second-order reaction. if the initial pressure of hl is 0.691 atm and
the rate constant for the reaction is 0.100 atm-1.min-1, how much
time (min) will pass before the hi pressure drops to 0.187 atm?
enter your answer to 1 decimal place.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 11:40
Which type of precipitation would most likely form when the surface air temperature is slightly below freezing and the air temperature increases as you move upward away from the ground?
Answers: 2


Chemistry, 23.06.2019 01:30
Which statement justifies that hydrogen peroxide (h2o2) is a polar molecule? the o – h bond is nonpolar and the molecule is asymmetric. the o – h bond is nonpolar and the molecule is symmetric. the o – h bond is polar and the molecule is asymmetric. the o – h bond is polar and the molecule is symmetric.
Answers: 1
You know the right answer?
Questions

Mathematics, 23.08.2021 01:50

Medicine, 23.08.2021 01:50


Mathematics, 23.08.2021 01:50

Mathematics, 23.08.2021 01:50


Mathematics, 23.08.2021 01:50




English, 23.08.2021 01:50

Mathematics, 23.08.2021 01:50



Mathematics, 23.08.2021 01:50

Mathematics, 23.08.2021 01:50



Mathematics, 23.08.2021 02:00

Mathematics, 23.08.2021 02:00

![\int\limits^{[HI_1]}_{[HI_0]} {\,\frac{d[HI]}{[HI]^2}=-\int\limits^t_0 {t} \, dt](/tpl/images/0222/4956/40b7f.png)
![\frac{1}{[HI_t]}-\frac{1}{[HI_0]}=kt](/tpl/images/0222/4956/6b6cb.png)