

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:50
Ase your answer to this question on the information below.hydrocarbons and fissionable nuclei are among the sources used for the production of energy in the united states. a chemical reaction produces much less energy than a nuclear reaction per mole of reactant.the balanced chemical equation below represents the reaction of one molecule of a hydrocarbon with two molecules of oxygen.chemical equation: ch4 + 2o2 → co2 + 2h2o + 1.48 × 10−18 jthe nuclear equation below represents one of the many possible reactions for one fissionable nucleus. in this equation, x represents a missing product.nuclear equation: write an isotopic notation for the missing product represented by x in the nuclear equation.
Answers: 1


Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 13:30
Apush or pull that moves or changes and object when to objects touch
Answers: 2
You know the right answer?
Suppose the amu had been defined as 110th of the mass of a phosphorus atom. what would be the relati...
Questions



Chemistry, 19.03.2020 16:07

Mathematics, 19.03.2020 16:07




Mathematics, 19.03.2020 16:09

Mathematics, 19.03.2020 16:09


History, 19.03.2020 16:09

History, 19.03.2020 16:10

Mathematics, 19.03.2020 16:10


Mathematics, 19.03.2020 16:14

Mathematics, 19.03.2020 16:14




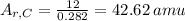
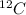 atom, thus according to the definition, the relative mass of carbon is exactly 12. If the definition of the AMU changed, we could calculate what the new relative mass is by dividing the relative mass of
atom, thus according to the definition, the relative mass of carbon is exactly 12. If the definition of the AMU changed, we could calculate what the new relative mass is by dividing the relative mass of 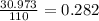 in old AMUs, if the atomic weight of carbon is 12.00, then in the new amu:
in old AMUs, if the atomic weight of carbon is 12.00, then in the new amu:

