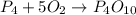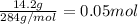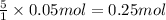Chemistry, 04.09.2019 05:20 vannybelly83
Given the unbalanced reaction: p₄+ o₂  p₄o₁₀ how many moles of o₂ are needed to produce 14.2 grams of p₄o₁₀?
p₄o₁₀ how many moles of o₂ are needed to produce 14.2 grams of p₄o₁₀?
a. 0.125 mol
b. 0.0620 mol
c. 0.500 mol
d. 0.0500 mol
e. 0.250 mol

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 3

Chemistry, 22.06.2019 13:00
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1

Chemistry, 22.06.2019 14:00
650.j is the same amount of energy as? 2720cal1550cal650.cal2.72cal
Answers: 2

You know the right answer?
Given the unbalanced reaction: p₄+ o₂ [tex]\rightarrow[/tex] p₄o₁₀ how many moles of o₂ are needed...
Questions

Chemistry, 28.12.2019 05:31






English, 28.12.2019 05:31


Mathematics, 28.12.2019 05:31




Social Studies, 28.12.2019 05:31

Computers and Technology, 28.12.2019 05:31


Mathematics, 28.12.2019 05:31


Arts, 28.12.2019 05:31

Mathematics, 28.12.2019 05:31

Biology, 28.12.2019 05:31