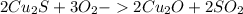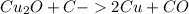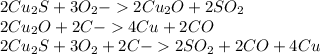Chemistry, 04.09.2019 04:10 trevorhenyan51
There are two steps in the extraction of copper metal from chalcocite, a copper ore. in the first step, copper(i) sulfide and oxygen react to form copper(i) oxide and sulfur dioxide. in the second step, copper(i) oxide and carbon react to form copper and carbon monoxide. write the net chemical equation for the production of copper from copper(i) sulfide, oxygen and carbon. be sure your equation is balanced.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 14:10
How does chemistry affect our world? a. chemicals makes our world more polluted. b. chemicals keeps us healthy. c. chemicals can or hurt our world. d. chemicals make our world safe to live in.
Answers: 1

Chemistry, 22.06.2019 04:30
In which phase(s) do the molecules take the shape of the container?
Answers: 1

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1

Chemistry, 22.06.2019 16:30
An atom with 7 protons, 6 neutrons, and 7 electrons has an atomic mass of amu. (enter a whole number.) numerical answers expected! answer for blank 1:
Answers: 3
You know the right answer?
There are two steps in the extraction of copper metal from chalcocite, a copper ore. in the first st...
Questions

History, 10.04.2021 19:50


Physics, 10.04.2021 19:50

Mathematics, 10.04.2021 19:50




Health, 10.04.2021 19:50




Mathematics, 10.04.2021 19:50

English, 10.04.2021 19:50


English, 10.04.2021 19:50

Mathematics, 10.04.2021 19:50


SAT, 10.04.2021 19:50

English, 10.04.2021 19:50

Mathematics, 10.04.2021 19:50

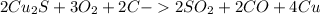
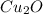 , that later reacts with C, to poduce the copper metal.
, that later reacts with C, to poduce the copper metal.