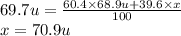Chemistry, 04.09.2019 03:20 hosteenimport21
The element gallium has an atomic mass of 69.7 u and consists of two stable isotopes gallium-69 and gallium-71. the isotope gallium-69 has a mass of 68.9 u and a percent natural abundance of 60.4 %. the isotope gallium-71 has a percent natural abundance of 39.6 %. what is the mass of gallium-71

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:30
As a part of an experiment a student burns propane to produce carbon dioxide and water she remembers that she must follow the law conservation of matter when writing a balanced chemical equation which of these equation adheres to the law of conservation of matter
Answers: 1

Chemistry, 21.06.2019 22:30
For the following, determine the type of reaction and then give products.
Answers: 2

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2

Chemistry, 22.06.2019 14:00
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3
You know the right answer?
The element gallium has an atomic mass of 69.7 u and consists of two stable isotopes gallium-69 and...
Questions


Mathematics, 19.02.2021 19:10


Mathematics, 19.02.2021 19:10

Mathematics, 19.02.2021 19:10


Mathematics, 19.02.2021 19:10

Spanish, 19.02.2021 19:10

Mathematics, 19.02.2021 19:10


Social Studies, 19.02.2021 19:10

History, 19.02.2021 19:10

Biology, 19.02.2021 19:10


Mathematics, 19.02.2021 19:10

Computers and Technology, 19.02.2021 19:10

Biology, 19.02.2021 19:10

Mathematics, 19.02.2021 19:20


Mathematics, 19.02.2021 19:20

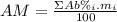
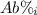 : percent natural abundance of each isotope
: percent natural abundance of each isotope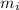 : mass of each isotope
: mass of each isotope