
Chemistry, 04.09.2019 02:30 peytonbrien2002
Pure magnesium metal is often found as ribbons and can easily burn in the presence of oxygen. when 4.51 g of magnesium ribbon burns with 6.92 g of oxygen, a bright, white light and a white, powdery product are formed. enter the balanced chemical equation for this reaction. be sure to include all physical states.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1


Chemistry, 22.06.2019 21:00
How many neutrons does an element have if its atomic number is 50 and its mass number is 166
Answers: 1

You know the right answer?
Pure magnesium metal is often found as ribbons and can easily burn in the presence of oxygen. when 4...
Questions

Mathematics, 29.09.2019 10:50


Mathematics, 29.09.2019 10:50


English, 29.09.2019 10:50

English, 29.09.2019 10:50

Health, 29.09.2019 10:50





History, 29.09.2019 10:50

History, 29.09.2019 10:50



Health, 29.09.2019 10:50


Biology, 29.09.2019 10:50

Physics, 29.09.2019 10:50

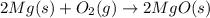
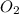 is present in gaseous state, and MgO is present in solid state.
is present in gaseous state, and MgO is present in solid state.

