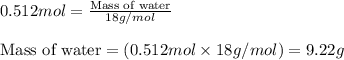Chemistry, 04.09.2019 02:30 dzepeda061
2n2h4(g)+ n2o4(g) à3 n2(g) + 4 h2o(g) when 8.0 g of n2h4(32 g mol-1) and 18.4 g of n2o4(92 g mol-1) are mixed together and react according to the equation above, what is the maximum mass of h2o that can be produced?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Which orbitals form a pi bond? a.the s orbital and three p orbitals b.the s orbital and two p orbitals c.overlapping p orbitals d.overlapping hybrid orbitals
Answers: 2

Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1

Chemistry, 22.06.2019 22:00
Imagine one batch of soup (batch “a”) is made with 8.19 g/can of salt, according to the recipe, and a second batch of soup (batch “b”) is made with 8.32 g/can of salt. explain which batch would be more resistant to frost damage if it is shipped a great distance in winter and explain why.
Answers: 2

Chemistry, 23.06.2019 02:00
What can be done to make a solid solute dissolve faster in a liquid solvent?
Answers: 1
You know the right answer?
2n2h4(g)+ n2o4(g) à3 n2(g) + 4 h2o(g) when 8.0 g of n2h4(32 g mol-1) and 18.4 g of n2o4(92 g mol-1)...
Questions




Mathematics, 06.08.2021 17:20

Mathematics, 06.08.2021 17:20


Biology, 06.08.2021 17:20




History, 06.08.2021 17:30

Business, 06.08.2021 17:30


Business, 06.08.2021 17:30

Mathematics, 06.08.2021 17:30




Computers and Technology, 06.08.2021 17:30

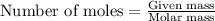 .....(1)
.....(1)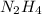 :
: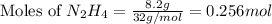
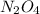 :
: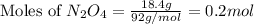
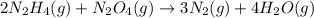
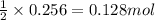 of
of 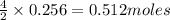 of water
of water