Chemistry, 04.09.2019 00:10 ashley5196
Sulfuric acid (h2so4) is prepared commercially from elemental sulfur using the contact process. in a typical sequence of reactions, the sulfur is first burned: s + o2 → so2 , then it is converted to so3 using a catalyst: 2 so2 + o2 → 2 so3 . the resulting so3 is reacted with water to produce the desired product: so3 + h2o → h2so4 . how much sulfuric acid could be prepared from 83 moles of sulfur? answer in units of g.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
The is a particle with one unit of positive charge a. proton b. positron c. electron d. nucleus awnser quick it is a important science test!
Answers: 2

Chemistry, 22.06.2019 08:00
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2

Chemistry, 22.06.2019 08:30
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1

Chemistry, 22.06.2019 22:30
Akno3 solution containing 51 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 3
You know the right answer?
Sulfuric acid (h2so4) is prepared commercially from elemental sulfur using the contact process. in a...
Questions

Mathematics, 03.12.2019 07:31

Mathematics, 03.12.2019 07:31



Health, 03.12.2019 07:31

Mathematics, 03.12.2019 07:31

Mathematics, 03.12.2019 07:31

English, 03.12.2019 07:31

Mathematics, 03.12.2019 07:31

English, 03.12.2019 07:31

History, 03.12.2019 07:31

Biology, 03.12.2019 07:31



Mathematics, 03.12.2019 07:31

Mathematics, 03.12.2019 07:31

History, 03.12.2019 07:31


Biology, 03.12.2019 07:31

Health, 03.12.2019 07:31

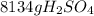
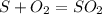
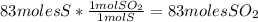
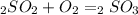
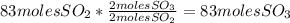
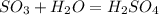
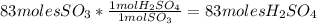
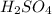 :
: