Chemistry, 03.09.2019 05:10 kjmccarty02
Determine the direction in which the following reaction is spontaneous at 25oc: mg2+(aq) + k(s) < > mg(s) + k+(aq) determine the equilibrium constant k and go using the cell potential for this reaction and which will be the anode and cathode?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:50
Which statement is a reason to support population regulation? a) it is unethical for us to control birth control rates b) humans have the freedom to produce as many children as desired c) the gap between the rich and poor has been narrowing since 1960 d) billions more people on the earth will intensify many environmental and social problems
Answers: 1

Chemistry, 22.06.2019 12:30
Clyde and marilyn are riding a roller coaster. during which section(s) of the track is their potential energy converted to kinetic energy? a. from point b to point c only b. from point b to point d only c. from point a to point b only d. from point a to point b and from point c to point d
Answers: 1

Chemistry, 22.06.2019 12:30
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1

Chemistry, 22.06.2019 14:30
What state of matter is ice a. liquid b. element c. solid d. gas
Answers: 1
You know the right answer?
Determine the direction in which the following reaction is spontaneous at 25oc: mg2+(aq) + k(s) <...
Questions

Computers and Technology, 02.06.2021 14:00



Mathematics, 02.06.2021 14:00


Mathematics, 02.06.2021 14:00

Arts, 02.06.2021 14:00

Health, 02.06.2021 14:00



Social Studies, 02.06.2021 14:00


English, 02.06.2021 14:00

Geography, 02.06.2021 14:00


Arts, 02.06.2021 14:00

Mathematics, 02.06.2021 14:00

Mathematics, 02.06.2021 14:00


Chemistry, 02.06.2021 14:00

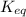 and
and 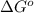 of the reaction is
of the reaction is 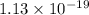 and -108080 J respectively.
and -108080 J respectively. 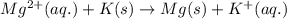
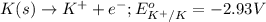
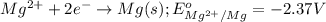
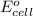 of the reaction, we use the equation:
of the reaction, we use the equation: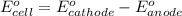
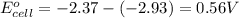
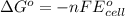
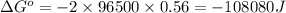
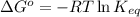
![25^oC=[273+25]=298K](/tpl/images/0221/6855/6a9f9.png)