
Which of the following states is true? (a) q does not change with temperature. (b) q is the same as keq when a reaction is at equilibrium. (c) key does not change with temperature, whereas q is temperature dependent. (d) k does not depend on the concentration or partial pressures of reaction components. (e) q does not depend on the concentrations or partial pressures of reaction components.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Asample of neon occupies a volume of 375 ml at stp. what will be the volume of neon if the pressure is reduced to 90.0 kpa? a. 422 ml b. 422 l c. 333 ml d. 333 l
Answers: 2

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and gas called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 18:00
Many pharmaceutical drugs are organic compounds that were originally synthesized in the laboratory. which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 2

Chemistry, 22.06.2019 22:30
How do limiting factors most affect population size? ostop population growthrestrict population growthincrease population sizeresult in positive impactso
Answers: 1
You know the right answer?
Which of the following states is true? (a) q does not change with temperature. (b) q is the same as...
Questions



Mathematics, 13.08.2020 23:01

Mathematics, 13.08.2020 23:01





Mathematics, 13.08.2020 23:01


Mathematics, 13.08.2020 23:01


Mathematics, 13.08.2020 23:01

Biology, 13.08.2020 23:01



Mathematics, 13.08.2020 23:01


Mathematics, 13.08.2020 23:01

English, 13.08.2020 23:01

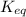 is the equilibrium constant and it is also equal to the concentration of products divided by the concentration of reactants. Also,
is the equilibrium constant and it is also equal to the concentration of products divided by the concentration of reactants. Also, 

