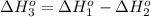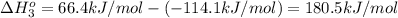Calculate the standard molar enthalpy of formation of no from the following data:
n2 + 2o2 -&...

Chemistry, 03.09.2019 03:30 babycakez143
Calculate the standard molar enthalpy of formation of no from the following data:
n2 + 2o2 -> 2no2 ho298 = 66.4kj
2no + o2 -> 2no2 ho298 = -114.1kj

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Needthe meter is the standard unit for: 1) height 2) length 3) weight 4) mass
Answers: 3

Chemistry, 22.06.2019 11:00
As air becomes more dense, (select all that apply) o. air weighs less o. gas molecules are closer together o. air is colder o. air weighs more o. gas molecules are further apart o. air is hotter
Answers: 3


Chemistry, 22.06.2019 19:00
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1
You know the right answer?
Questions

Mathematics, 03.08.2019 04:30

History, 03.08.2019 04:30

History, 03.08.2019 04:30



Computers and Technology, 03.08.2019 04:30

Biology, 03.08.2019 04:30

Social Studies, 03.08.2019 04:30



Physics, 03.08.2019 04:30


Mathematics, 03.08.2019 04:30







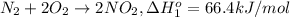 ..(1)
..(1) ..(2)
..(2)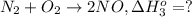 ..(3)
..(3)