
Chemistry, 03.09.2019 03:30 tilly40oooo
The half-life for the second-order decomposition of hi is 15.4 s when the initial concentration of hi is 0.67 m. what is the rate constant for this reaction? a) 1.0 * 10-2 m-15-1 b) 4.5 * 10-2 m-15-1 c) 9.7* 10-2 m-15-1 od) 2.2 * 10-2 m-15-1

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2

Chemistry, 22.06.2019 15:30
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3

Chemistry, 22.06.2019 22:30
Which of the following is true about the speed of light? it depends on the wavelength.
Answers: 3
You know the right answer?
The half-life for the second-order decomposition of hi is 15.4 s when the initial concentration of h...
Questions


Chemistry, 21.09.2019 02:30

Physics, 21.09.2019 02:30


Mathematics, 21.09.2019 02:30

Mathematics, 21.09.2019 02:30






Mathematics, 21.09.2019 02:30


Biology, 21.09.2019 02:30






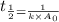
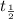 = half life = 15.4 s
= half life = 15.4 s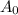 = initial concentration = 0.67 M
= initial concentration = 0.67 M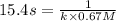
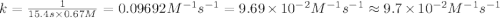
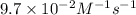 is the rate constant for this reaction.
is the rate constant for this reaction.

