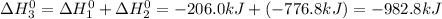Calculate ho298 for the process zn + s + 2o2 ? znso4 from the following information:
zn + s...


Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 20:20
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1


Chemistry, 23.06.2019 04:00
What is the volume of 2.5 moles of nitrogen gas (n2)at standard temperature and pressure (stp)?
Answers: 1

Chemistry, 23.06.2019 07:30
If you try to move a piano and are unable to move it, did you perform any work in the scientific sense of the word? yes no correct anwser get brainliest
Answers: 1
You know the right answer?
Questions


English, 12.01.2020 17:31

History, 12.01.2020 17:31

History, 12.01.2020 17:31










Social Studies, 12.01.2020 17:31

Mathematics, 12.01.2020 17:31


Mathematics, 12.01.2020 17:31


Mathematics, 12.01.2020 17:31

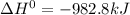 .
.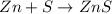
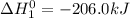 (1)
(1)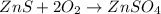
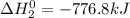 (2)
(2)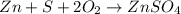
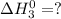 (3)
(3)