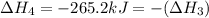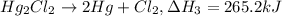Calculate h for the process hg2cl2 ? 2hg + cl2 from the following information:
hg + cl2 --&g...

Chemistry, 03.09.2019 03:30 gonzmari457
Calculate h for the process hg2cl2 ? 2hg + cl2 from the following information:
hg + cl2 --> hgcl2 h = -224kj
hg + hgcl2 --> hg2cl2 h =-41.2 kj

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
The p sub shell can hold up to 8 electrons in an atom. true or false?
Answers: 1

Chemistry, 22.06.2019 05:20
Why does the sun appear to be the brightest star in the sky? a- its apparent brightness is much greater than other stars. b- it burns more gas, making it brighter than any other star. c- it is the largest star in the galaxy, so it is the brightest star. d- its relative distance to earth is closer than the other stars.
Answers: 1


Chemistry, 22.06.2019 13:30
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2
You know the right answer?
Questions



Mathematics, 10.12.2020 09:30



Mathematics, 10.12.2020 09:30

Geography, 10.12.2020 09:30



Mathematics, 10.12.2020 09:30



World Languages, 10.12.2020 09:30


Chemistry, 10.12.2020 09:30


Mathematics, 10.12.2020 09:30

Biology, 10.12.2020 09:30

History, 10.12.2020 09:30

Mathematics, 10.12.2020 09:30

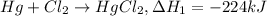 ...(1)
...(1)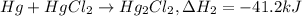 ..(2)
..(2)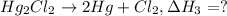 ...(3)
...(3)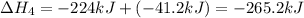
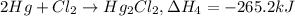 ...(4)
...(4)