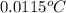Chemistry, 03.09.2019 02:30 12martinkat
How much heat is produced when 100ml of 0.25 m hcl (density 1.00g/ml) and 200 ml of 0.150 m naoh (densty 1.00g/ml) are mixed?
hcl + naoh ? nacl + h2o ho298= -58kj
if both solutions are the same temperatureand heat capaciy of the products is 4.19 j/gc, how much will the temperature increase? what assumption did you make in your calculation?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 10:30
Find the number of grams of hcl needed to react completely with .50 moles of magnesium.
Answers: 1

Chemistry, 22.06.2019 15:30
Two metal blocks that have slightly different temperatures are placed next to one another. after five minutes, they both have lower but equal temperatures. according to the law of conservation of energy, what most likelyhappened? energy was created inside the blocks.energy was destroyed inside the blocks.energy was absorbed into the blocks from outside the system.energy was transferred from the warmer block to the cooler block.
Answers: 2

Chemistry, 23.06.2019 00:00
What does an electron configuration for an atom relate to the atoms placement on the periodic table
Answers: 2
You know the right answer?
How much heat is produced when 100ml of 0.25 m hcl (density 1.00g/ml) and 200 ml of 0.150 m naoh (de...
Questions


Mathematics, 07.12.2019 09:31

Health, 07.12.2019 09:31


English, 07.12.2019 09:31




Mathematics, 07.12.2019 09:31



Physics, 07.12.2019 09:31

Biology, 07.12.2019 09:31

History, 07.12.2019 09:31


History, 07.12.2019 09:31

Biology, 07.12.2019 09:31

Mathematics, 07.12.2019 09:31

Mathematics, 07.12.2019 09:31

Mathematics, 07.12.2019 09:31

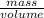
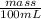
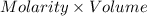
 (as 1 ml = 1000 L)
(as 1 ml = 1000 L)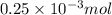
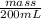
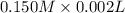 (as 1 ml = 1000 L)
(as 1 ml = 1000 L)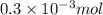
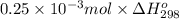
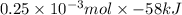
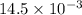 kJ
kJ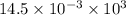 J
J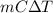
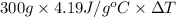
 =
=