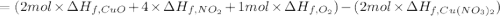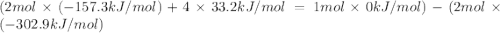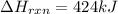The decomposition of copper(ii) nitrate on heating is endothermic reaction. 2cu(no3)2(s) → 2c10(s) + 4no2(g) + o2(g) calculate the enthalpy change for this reaction using the following enthalpy changes of formation. ah! [cu(no3)2) = -302.9 kj mol? ah, (cuo) = -157.3 kj mol? . ah[no2) = +33.2 kj mol.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Start an single atom tab. observe the decay of polonium-211. after each decay, press the reset nucleus button to watch the process again. write a description of alpha decay for po-211
Answers: 2

Chemistry, 22.06.2019 01:40
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1

Chemistry, 22.06.2019 15:20
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3
You know the right answer?
The decomposition of copper(ii) nitrate on heating is endothermic reaction. 2cu(no3)2(s) → 2c10(s) +...
Questions

Mathematics, 25.02.2020 04:45


Mathematics, 25.02.2020 04:45


Biology, 25.02.2020 04:46

Mathematics, 25.02.2020 04:46



Mathematics, 25.02.2020 04:46







Mathematics, 25.02.2020 04:46

Mathematics, 25.02.2020 04:46

Computers and Technology, 25.02.2020 04:46


Mathematics, 25.02.2020 04:47

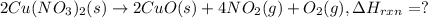
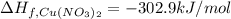
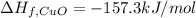
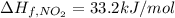
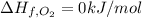 (standard state)
(standard state)![\Delta H_{rxn}=\sum [\Delta H_f(product)]-\sum [\Delta H_f(reactant)]](/tpl/images/0221/5790/84aad.png)
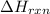 =
=