Chemistry, 03.09.2019 00:10 thedragontale1020
Calculate for the following electrochemical cell at 25°c, pt h2(g) (1.0 atm) h (0.010 m || ag (0.020 m) ag if e (h) - +0.000 v and e(ag)-0.799 v. a. +0.817 v b. +0.799 v c. +0.911 v d. +0.275 v e. +1.01 v ,

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:50
What is the overall order of reaction for rate = k[no2]2 ? second order 3/2 order third order zero order none of the listed answers are correct
Answers: 3

Chemistry, 22.06.2019 05:30
Transportation is the largest single source of air pollution in the united states. air pollution can harm the environment and human health. which technology could offer a solution to this problem? mufflers that reduce noise motors that run on electricity tires that improve gas mileage
Answers: 3

Chemistry, 22.06.2019 12:40
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1

You know the right answer?
Calculate for the following electrochemical cell at 25°c, pt h2(g) (1.0 atm) h (0.010 m || ag (0.020...
Questions




Arts, 21.07.2019 14:00




Computers and Technology, 21.07.2019 14:00

Spanish, 21.07.2019 14:00

Mathematics, 21.07.2019 14:00

Social Studies, 21.07.2019 14:00

English, 21.07.2019 14:00


Computers and Technology, 21.07.2019 14:00


Mathematics, 21.07.2019 14:00


Physics, 21.07.2019 14:00

History, 21.07.2019 14:00

History, 21.07.2019 14:00

![E^0_{[H^{+}/H_2]}=+0.00V](/tpl/images/0221/4856/30abe.png)
![E^0_{[Ag^{+}/Ag]}=+0.799V](/tpl/images/0221/4856/2c999.png)
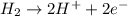
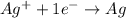
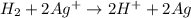
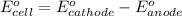
![E^o_{cell}=E^o_{[Ag^{+}/Ag]}-E^o_{[H^{+}/H_2]}](/tpl/images/0221/4856/e75b5.png)