

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
An alkaline battery produces electrical energy according to the following equation. zn(s) + 2 mno2(s) + h2o(l) zn(oh)2(s) + mn2o3(s) (a) determine the limiting reactant if 17.5 g zn and 31.0 g mno2 are used. (type your answer using the format ch4 for ch4.) (b) determine the mass of zn(oh)2 produced. _ g
Answers: 3


Chemistry, 22.06.2019 20:10
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3

Chemistry, 22.06.2019 22:30
Which of these statements best explains why space exploration should be encouraged? it prepares humans to live without oxygen. it dispel myths about objects in space. it prevents comets and asteroids from striking earth. it creates technology to absorb harmful radiations in space.
Answers: 1
You know the right answer?
Magnesium will burn in air to form both mg3n2 and mgo. what mass of each product would be found if b...
Questions


Mathematics, 02.12.2020 22:00

Mathematics, 02.12.2020 22:00


History, 02.12.2020 22:00

Mathematics, 02.12.2020 22:00

Mathematics, 02.12.2020 22:00


Biology, 02.12.2020 22:00

Mathematics, 02.12.2020 22:00

Biology, 02.12.2020 22:00

Mathematics, 02.12.2020 22:00

English, 02.12.2020 22:00



Social Studies, 02.12.2020 22:00

English, 02.12.2020 22:00



Mathematics, 02.12.2020 22:00

 formed is 1.587 grams.
formed is 1.587 grams. .....(1)
.....(1)
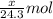
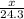 moles of magnesium will produce
moles of magnesium will produce 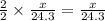 moles of MgO
moles of MgO
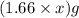 = X grams
= X grams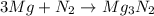

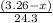 moles of magnesium will produce
moles of magnesium will produce 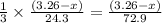 moles of
moles of ![Mg_3N_2=\frac{(3.26-x)}{72.9}\times 101=[(3.26-x)\times 1.38]g](/tpl/images/0221/4247/e57b9.png)
![[(3.26-x)\times 1.38]g](/tpl/images/0221/4247/79581.png) = Y grams
= Y grams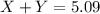
![(1.66\times x)+[(3.26-x)\times 1.38]=5.09\\\\x=2.11g](/tpl/images/0221/4247/08016.png)
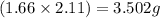
![Mg_3N_2=[(3.26-2.11)\times 1.38]=1.587g](/tpl/images/0221/4247/42984.png)


