Chemistry, 02.09.2019 22:20 Jackiecroce12
H2 is produced by the reaction of 118.5 ml of a 0.8775-m solution of h3po4 according to the following equations: 2cr +
2h3po4 ? 3h2 + 2crpo4.
(a) outline the steps neccessary to determine the number of moles and mass of h2
(b)perform the calculations outlined

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 11:30
Aperfume bottle is dropped in the corner of a room. the odor of the perfume can be detected on the other side of the room. which statement best describes this observation?
Answers: 2

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2

Chemistry, 22.06.2019 20:00
In vapor-liquid equilibrium in a binary mixture, both components are generally present in both phases. how many degrees of freedom are there for such a system? the reaction between nitrogen and hydrogen to form ammonia occurs in the gas phase. how many degrees of freedom are there for this system? steam and coal react at high temperatures to form hydrogen, carbon monoxide, carbon dioxide, and methane. the following reactions have been suggested as being involved in the chemical transformation:
Answers: 3
You know the right answer?
H2 is produced by the reaction of 118.5 ml of a 0.8775-m solution of h3po4 according to the followin...
Questions


Mathematics, 23.11.2020 14:00






Spanish, 23.11.2020 14:00


Mathematics, 23.11.2020 14:00

History, 23.11.2020 14:00

Social Studies, 23.11.2020 14:00

English, 23.11.2020 14:00

English, 23.11.2020 14:00

Chemistry, 23.11.2020 14:00

Business, 23.11.2020 14:00

Mathematics, 23.11.2020 14:00

Physics, 23.11.2020 14:00


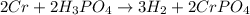
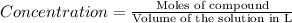

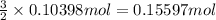 of hydrogen gas
of hydrogen gas