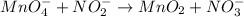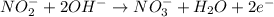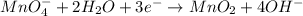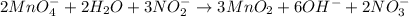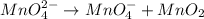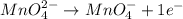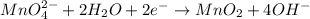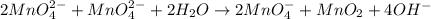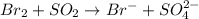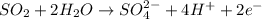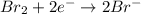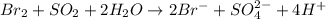Chemistry, 02.09.2019 22:10 dbn4everloved8
Balance each of the following equations according to the half- reaction method:
(a) mno4- + no2- --> mno2 + no3- (in base)
(b) mno42- --> mno4- +mno2 (in base)
(c) br2 + so2 --> br- + so42- (in acid)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Idon't really understand this can you me and show your work.☺☺[ chemistry b] subject [ electron transfer in lonic bonds]grade( 12)
Answers: 1

Chemistry, 22.06.2019 12:30
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2

Chemistry, 22.06.2019 22:30
Akno3 solution containing 51 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 3

You know the right answer?
Balance each of the following equations according to the half- reaction method:
(a) mno4- + n...
(a) mno4- + n...
Questions

Health, 23.11.2020 18:30

Mathematics, 23.11.2020 18:30


Social Studies, 23.11.2020 18:30

Mathematics, 23.11.2020 18:30


Computers and Technology, 23.11.2020 18:40


World Languages, 23.11.2020 18:40



English, 23.11.2020 18:40


Mathematics, 23.11.2020 18:40


Social Studies, 23.11.2020 18:40

Mathematics, 23.11.2020 18:40


Mathematics, 23.11.2020 18:40

English, 23.11.2020 18:40