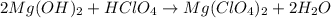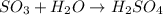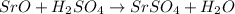Chemistry, 02.09.2019 21:10 jesusmojica25
Complete and balance the equation for the following acid-base neutralization reaction. if water is used as a solvent, write the reactants and products as aqueous ions. in some cases, there may be more than one correct answer, depending on the amount of reactants used.
(a)mg(oh)2 + hclo4?
(b)so3 + h2o? (assume an excess of water and that the product dissolves)
(c) sro + h2so4?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which step in naming unsaturated hydrocarbons is used for alkenes but not alkynes
Answers: 2


Chemistry, 22.06.2019 17:20
Pegmatites are igneous rocks in which the individual minerals are very large. typically, the minerals are all light-colored quartz, feldspar and muscovite. if you were given a black and white photograph of a pegmatite in a quarry (where the rock has been blasted and broken), what physical properties could you use to identify those three minerals in this hypothetical photo? describe each mineral and the specific diagnostic properties. be specific.
Answers: 2

Chemistry, 22.06.2019 19:00
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1
You know the right answer?
Complete and balance the equation for the following acid-base neutralization reaction. if water is u...
Questions

Chemistry, 20.05.2021 06:50


History, 20.05.2021 06:50



Mathematics, 20.05.2021 06:50

Mathematics, 20.05.2021 06:50


Mathematics, 20.05.2021 06:50

Mathematics, 20.05.2021 06:50


Health, 20.05.2021 06:50


Mathematics, 20.05.2021 06:50

Health, 20.05.2021 06:50

Mathematics, 20.05.2021 06:50


Mathematics, 20.05.2021 06:50

History, 20.05.2021 06:50

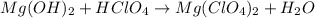
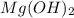 by 2 on reactant side and multiply
by 2 on reactant side and multiply 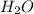 by 2 on product side.
by 2 on product side.