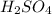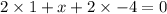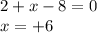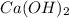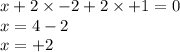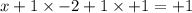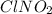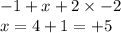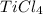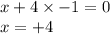Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
What is the number of moles of chemical units represented by 9.03x10^24? and how do i show work? (dumb it down )
Answers: 1

Chemistry, 22.06.2019 13:30
Which of the following has wavelength longer than the wavelength of viable light? a) x rays b) gamma rays c) radios waves d) ultraviolet waves
Answers: 1

Chemistry, 22.06.2019 14:10
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1

Chemistry, 22.06.2019 19:00
What information does a complete ionic equation give that the balanced equation doesn’t show?
Answers: 1
You know the right answer?
Determine the oxidation states of the elements in the compounds listed. none of the oxygen-containin...
Questions



Computers and Technology, 24.03.2020 01:13



Mathematics, 24.03.2020 01:13


Geography, 24.03.2020 01:13




Mathematics, 24.03.2020 01:14

Mathematics, 24.03.2020 01:14


Mathematics, 24.03.2020 01:14

Mathematics, 24.03.2020 01:14



Computers and Technology, 24.03.2020 01:14

History, 24.03.2020 01:14