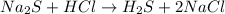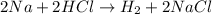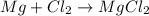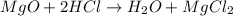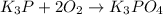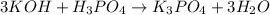Chemistry, 02.09.2019 20:30 makaylarae8781
Classify the following as acid-base reactions or oxidation-reduction reactions:
(a)na2s + hcl -> h2s + 2nacl
(b)2na + 2hcl -> h2 + 2nacl
(c)mg + cl2 = mgcl2
(d)mgo + 2hcl = h2o + mgcl2
(e)k3p+2o2 -> k3po4
(f)3koh +h3po4 -> k3po4 + 3h2o

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:30
150.0 grams of asf3 were reacted with 180.0 g of ccl4 to produce ascl3 and ccl2f2. if the actual yield of ccl2f2 was 127 g, what is the percent yield?
Answers: 2

Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3


Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2
You know the right answer?
Classify the following as acid-base reactions or oxidation-reduction reactions:
(a)na2s + hcl...
(a)na2s + hcl...
Questions

English, 17.12.2020 16:30

Arts, 17.12.2020 16:30



Mathematics, 17.12.2020 16:30



History, 17.12.2020 16:30

Mathematics, 17.12.2020 16:30









Mathematics, 17.12.2020 16:40

Mathematics, 17.12.2020 16:40