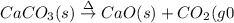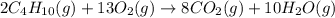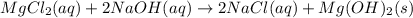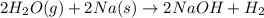Chemistry, 02.09.2019 20:10 codyshs160
Write a balanced molecular equation describing each of the following chemical reactions:
(a)solid calcium carbonate is heated and decomposes to sold calcium oxide and carbon dioxide
(b)gaseous butane, c4h10, reacts with diatomic oxygen gas to yield gaseous carbon dioxide and water vapor
(c)aaqeous solutions of magnesium chloride and sodium hydroxide react to produce solid magnesium hydroxide and aqueous sodium chloride
(d)water vapor reacts with sodium metal to produce solid sodium hydroxide and hydrogen gas

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which type of bond is present in hydrogen sulfide (h2s)? the table of electronegativities is given. a. hydrogen b. ionic c. nonpolar covalent d. polar covalent
Answers: 1

Chemistry, 22.06.2019 04:00
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution?
Answers: 2


Chemistry, 22.06.2019 19:20
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3
You know the right answer?
Write a balanced molecular equation describing each of the following chemical reactions:
(a)s...
(a)s...
Questions







History, 14.03.2020 06:19

Mathematics, 14.03.2020 06:19

Mathematics, 14.03.2020 06:19


Social Studies, 14.03.2020 06:19


English, 14.03.2020 06:19



Social Studies, 14.03.2020 06:20


Biology, 14.03.2020 06:20

Mathematics, 14.03.2020 06:20