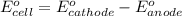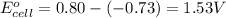Use the following half-reactions to construct a voltaic cell:
cr3+(aq) + 3e−\rightarrow→ cr(s...

Chemistry, 02.09.2019 19:20 bayleeharris8p78txa
Use the following half-reactions to construct a voltaic cell:
cr3+(aq) + 3e−\rightarrow→ cr(s) eo = −0.73 v
ag+(aq) + e−\rightarrow→ ag(s) eo = 0.80 v
determine the balanced overall redox reaction, and calculate eocell.
3 ag(s) + cr3+(aq) \rightarrow→ 3 ag+(aq) + cr(s), eocell = −1.53 v
3 ag+(aq) + cr(s) \rightarrow→ 3 ag(s) + cr3+(aq),eocell = +3.13 v
3 ag(s) + cr3+(aq) \rightarrow → 3 ag+(aq) + cr(s), eocell = −3.13 v
ag(s) + cr3+(aq) \rightarrow→ ag+(aq) + cr(s), eocell = −1.53 v
3 ag+(aq) + cr(s) \rightarrow→ 3 ag(s) + cr3+(aq), eocell = +1.53 v
ag+(aq) + cr(s) \rightarrow→ ag(s) + cr3+(aq), eocell = +1.53 v

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 22.06.2019 14:30
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1


Chemistry, 23.06.2019 00:30
How many moles of co2 are produced during the complete combustion of 3.6 moles of c2h6
Answers: 1
You know the right answer?
Questions












Biology, 10.03.2020 04:41








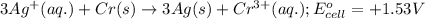
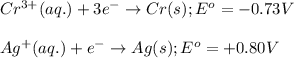
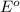 potential will always get reduced and will undergo reduction reaction. Here, silver will always undergo reduction reaction will get reduced.
potential will always get reduced and will undergo reduction reaction. Here, silver will always undergo reduction reaction will get reduced.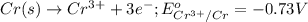
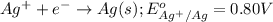 ( × 3)
( × 3)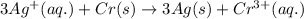
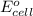 of the reaction, we use the equation:
of the reaction, we use the equation: