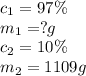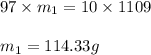Chemistry, 02.09.2019 19:20 puppylover72
What mass of solid naoh (97.0 % by mass) is required to prepare 1.00 l of a 10.0% solution of naoh by mass? the density of the 10.0% solution is 1.109 g/ml.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:50
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3

Chemistry, 22.06.2019 16:40
Identify the lewis acid in this balanced equation: ag+ + 2nh3 -> ag(nh3)2+a. ag+b. nh3c. ag(nh3)2+
Answers: 1

Chemistry, 22.06.2019 18:50
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3

Chemistry, 23.06.2019 00:30
•hydration •dissociation •dissolving which one goes to which
Answers: 1
You know the right answer?
What mass of solid naoh (97.0 % by mass) is required to prepare 1.00 l of a 10.0% solution of naoh b...
Questions


World Languages, 05.03.2021 18:50



Biology, 05.03.2021 18:50

English, 05.03.2021 18:50

Mathematics, 05.03.2021 18:50




Computers and Technology, 05.03.2021 18:50


Mathematics, 05.03.2021 18:50

English, 05.03.2021 18:50


Mathematics, 05.03.2021 18:50

Arts, 05.03.2021 18:50

English, 05.03.2021 18:50

Biology, 05.03.2021 18:50

Chemistry, 05.03.2021 18:50

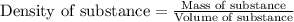
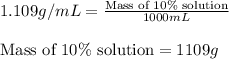
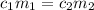
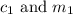 are the concentration and mass of concentrated solution.
are the concentration and mass of concentrated solution.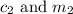 are the concentration and mass of diluted solution.
are the concentration and mass of diluted solution.