
Calculate the molarity of each of the following solutions:
(a) 293 gram hcl in 666 ml of solution, a concentrated hcl solution
(b)2.026 g in 0.1250 ml of a solution used as an unknown in general chemistry laboratory
(c) 0.001 mg cd2+ in 0.100 l the maximum permissible concentration of cadmium in drinking water
(d) 0.0079 g c7h5sno3 in one ounce(29.6ml), concentration of saccharin in a diet soft drink

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 17:00
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1

Chemistry, 23.06.2019 19:30
The melting point of an ionic compound is likely to be blank and michael molecula the melting point of an ionic compound is likely to be a molecular compound
Answers: 3

Chemistry, 24.06.2019 06:30
What represents the different forms of electromagnetic radiation
Answers: 1

You know the right answer?
Calculate the molarity of each of the following solutions:
(a) 293 gram hcl in 666 ml of solu...
(a) 293 gram hcl in 666 ml of solu...
Questions

Mathematics, 09.04.2021 17:50

Biology, 09.04.2021 17:50

Mathematics, 09.04.2021 17:50

Mathematics, 09.04.2021 17:50

History, 09.04.2021 17:50



Mathematics, 09.04.2021 17:50





Social Studies, 09.04.2021 17:50


Mathematics, 09.04.2021 17:50



History, 09.04.2021 17:50

Mathematics, 09.04.2021 17:50

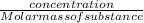
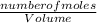
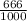 dm³ , 0.666dm³
dm³ , 0.666dm³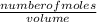
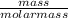
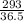 = 8.03mole
= 8.03mole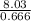 = 12.06moldm⁻³
= 12.06moldm⁻³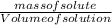 =
= 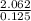 = 16.496g/mL
= 16.496g/mL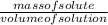 =
= 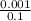 = 0.01mg/L
= 0.01mg/L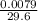 = 0.00027g/mL
= 0.00027g/mL


