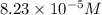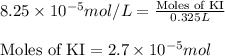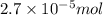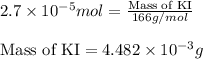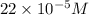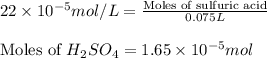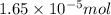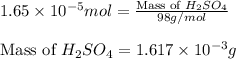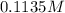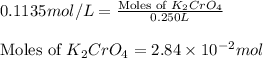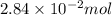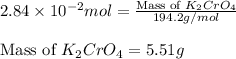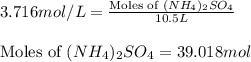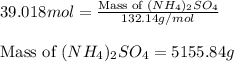Calculate the number of moles and the mass of the solute in each of the following solutions:
(a) 325 ml of 8.23 x 10-5 m kl, a source of iodine in the diet
(b) 75.0 ml of 22 x 10-5 m h2so4, a sample of acid rain
(c) 0.2500 l of 0.1135 m k2cro4 and analytical reagent used in iron assays
(d)10.5 l of 3.716 m (nh4)2so4, a liquid fertilizer

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:40
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3

Chemistry, 22.06.2019 12:00
An atom's configuration based on its number of electrons ends at 3p4. another atom has seven more electrons. starting at 3p, what is the remaining configuration? 3p63d34s2 3p43d54s2 3p64s23d3 3p44s23d
Answers: 3

Chemistry, 22.06.2019 18:00
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3

Chemistry, 22.06.2019 19:40
What causes different colors to appear in the sky? the absorption of light by air molecules the reflection of light by bodies of water the greenhouse effect in earth's atmosphere the scattering and reflection of light by dust particles
Answers: 2
You know the right answer?
Calculate the number of moles and the mass of the solute in each of the following solutions:
...
...
Questions

Chemistry, 20.11.2020 17:20


Chemistry, 20.11.2020 17:20

Mathematics, 20.11.2020 17:20




Engineering, 20.11.2020 17:20


Advanced Placement (AP), 20.11.2020 17:20


Mathematics, 20.11.2020 17:30

Mathematics, 20.11.2020 17:30


Mathematics, 20.11.2020 17:30


Biology, 20.11.2020 17:30


Mathematics, 20.11.2020 17:30

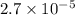 and mass is
and mass is 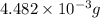
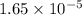 and mass is
and mass is 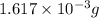
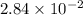 and mass is 5.51 g.
and mass is 5.51 g.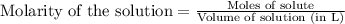 .....(1)
.....(1)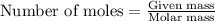 .....(2)
.....(2)