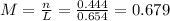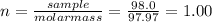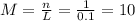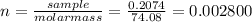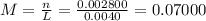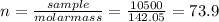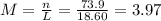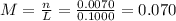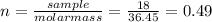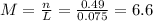Chemistry, 02.09.2019 18:20 nyraimccall408
Determine the molarity for each of the following solution solutions:
(a)0.444 mol of cocl2 in 0.654 l of solution
(b) 98.0 gram of phosphoric acid, h3po4, in 100 l of solution
(c) 0.2074 g of calcium hydroxide, ca(oh)2 in 40.00 ml of solution
(d)10.5 kg of na2ao4.10h2o in 18.60 l of solution
(e) 7.0 x 10-3 mol of l2 in 100.0 ml of solution
(f) 1.8 x 104 mg of hcl in 0.075 of solution

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
How do i complete this electrolysis of water lab? i’m at home, so i don’t have the materials, and the lab didn’t properly work and was incomplete at school.
Answers: 1

Chemistry, 22.06.2019 14:50
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1

Chemistry, 22.06.2019 20:20
Which symbol can be used to indicate the pressure at which a chemical reaction is carried out? 25°c 2 atm pa
Answers: 2

You know the right answer?
Determine the molarity for each of the following solution solutions:
(a)0.444 mol of cocl2 i...
(a)0.444 mol of cocl2 i...
Questions


Mathematics, 10.09.2019 03:30



Mathematics, 10.09.2019 03:30

English, 10.09.2019 03:30


Advanced Placement (AP), 10.09.2019 03:30

Mathematics, 10.09.2019 03:30






Biology, 10.09.2019 03:30