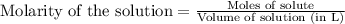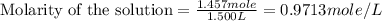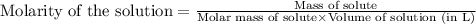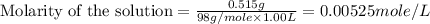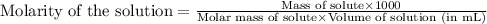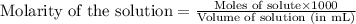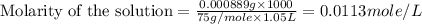Chemistry, 02.09.2019 18:20 aubreyfoster
Determine the molarity for each of the following solution solutions:
(a)1.457 mol of kcl in 1.500 l of solution
(b) 0.515 gram ofh2so4, in 1.00 l of solution
(c) 20.54 g of al(no3)3 in 1575 ml of solution
(d)2.76 kg ofcuso4.5h2o in 1.45 l of solution
(e)0.005653 mol ofbr2 in 10.00 ml of solution
(f) 0.000889 g of glycine, c2h5no2, in 1.05 ml of solution

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

Chemistry, 22.06.2019 06:00
What type of electromagnetic radiation has a shorter wavelength than blue light
Answers: 2

Chemistry, 22.06.2019 10:40
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1

Chemistry, 22.06.2019 18:00
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3
You know the right answer?
Determine the molarity for each of the following solution solutions:
(a)1.457 mol of kcl in...
(a)1.457 mol of kcl in...
Questions


Mathematics, 10.03.2021 01:00



Mathematics, 10.03.2021 01:00

Mathematics, 10.03.2021 01:00

Chemistry, 10.03.2021 01:00






History, 10.03.2021 01:00

English, 10.03.2021 01:00


Mathematics, 10.03.2021 01:00

English, 10.03.2021 01:00

Mathematics, 10.03.2021 01:00

Mathematics, 10.03.2021 01:00

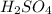 solution is, 0.00525 mole/L
solution is, 0.00525 mole/L solution is, 0.0612 mole/L
solution is, 0.0612 mole/L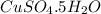 solution is, 7.61 mole/L
solution is, 7.61 mole/L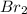 solution is, 0.0565 mole/L
solution is, 0.0565 mole/L solution is, 0.0113 mole/L
solution is, 0.0113 mole/L