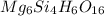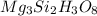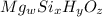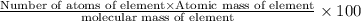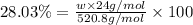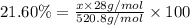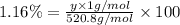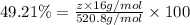Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:00
How are ionic bonds formed and what is the attractive force within an ionic bond
Answers: 1

Chemistry, 22.06.2019 02:10
3.) for each of the following compounds, draw the major organic product of reaction with hcl or naoh and circle whether the starting materials and products will be more soluble in organic solvent or water benzoic acid + hcl: benzoic acid + naoh: oh benzoic acid water/organic water organic fluorenone hс: fluorenone + naoh: fluorenone water/organic water/organic веnzocaine + hci: benzocaine + n»oh: h2n benzocaine water/organic water organic o=
Answers: 3

Chemistry, 22.06.2019 02:30
When svante arrhenius first proposed his acid-base theory, he was a doctoral candidate. his professors thought his ideas were unfounded. within a decade, the arrhenius theory of acid-base was widely accepted and praised within the scientific world. arrhenius defined acids as compounds having ionizable hydrogen and bases as compounds with ionizable a) barium. b) hydronium. c) hydroxide. d) oxygen.
Answers: 3

You know the right answer?
Determining the empirical and molecular formula for chyrsotile asbestos. chyrsotile has the followin...
Questions

Computers and Technology, 27.06.2019 05:40

English, 27.06.2019 05:40

Biology, 27.06.2019 05:40

English, 27.06.2019 05:40


Mathematics, 27.06.2019 05:40

Advanced Placement (AP), 27.06.2019 05:40


Mathematics, 27.06.2019 05:40

Physics, 27.06.2019 05:40

Mathematics, 27.06.2019 05:40



Mathematics, 27.06.2019 05:40


Mathematics, 27.06.2019 05:40

Chemistry, 27.06.2019 05:40

Social Studies, 27.06.2019 05:40

Social Studies, 27.06.2019 05:40