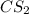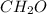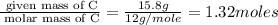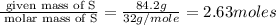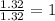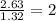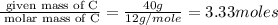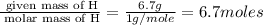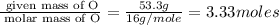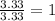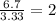Determine the empirical formulas for compounds with the following percent compositions:
(a)15...


Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Asample of the male sex hormone testosterone, c19h28o2, contains 3.88×10^21 atoms of hydrogen.(a) how many atoms of carbon does it contain? (b) how many molecules of testosterone does it contain? (c) how many moles of testosterone does it contain? (d) what is the mass of this sample in grams?
Answers: 1

Chemistry, 21.06.2019 20:00
Different isotopes indicate that an element will have different numbers of
Answers: 2

Chemistry, 21.06.2019 23:00
Achef makes salad dressing by mixing oil, vinegar, and spices, as shown. which type of matter is the salad dressing?
Answers: 1

Chemistry, 22.06.2019 00:00
Which of the following statements is true? a. elements in the last period are radioactive. b. atomic weight is the same as atomic mass. c. elements in the same group have the same number of electron shells. d. atomic number equals the number of neutrons in the nucleus of an atom.
Answers: 1
You know the right answer?
Questions




History, 04.02.2021 22:00

English, 04.02.2021 22:00

Mathematics, 04.02.2021 22:00



Mathematics, 04.02.2021 22:00

Mathematics, 04.02.2021 22:00



Spanish, 04.02.2021 22:00



Mathematics, 04.02.2021 22:00

Mathematics, 04.02.2021 22:00

History, 04.02.2021 22:00

Mathematics, 04.02.2021 22:00

English, 04.02.2021 22:00