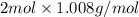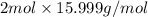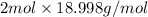Chemistry, 02.09.2019 16:10 alexius6608
Compare 1 mole of h2, 1 mole of o2 and 1 mole of f2
(a)which has the largest number of molecule? explain why
(b)which has the greatest mass? explain why

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:50
Ase your answer to this question on the information below.hydrocarbons and fissionable nuclei are among the sources used for the production of energy in the united states. a chemical reaction produces much less energy than a nuclear reaction per mole of reactant.the balanced chemical equation below represents the reaction of one molecule of a hydrocarbon with two molecules of oxygen.chemical equation: ch4 + 2o2 → co2 + 2h2o + 1.48 × 10−18 jthe nuclear equation below represents one of the many possible reactions for one fissionable nucleus. in this equation, x represents a missing product.nuclear equation: write an isotopic notation for the missing product represented by x in the nuclear equation.
Answers: 1

Chemistry, 22.06.2019 11:30
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2

Chemistry, 22.06.2019 12:00
What are the first two quantum numbers for the electrons located in subshell 4d? what are the first three quantum numbers for the electrons located in subshell 2s? how many electrons can be held in a sublevel l = 3? how many electrons can be held in the energy level n = 4? how many electrons in an atom can share the quantum numbers n = 4 and l = 3?
Answers: 1

Chemistry, 22.06.2019 22:00
The volume of an unknown substance in a sealed glass jar is 50 milliliters. the volume of the jar is 200 milliliters. which state of matter could the substance be?
Answers: 2
You know the right answer?
Compare 1 mole of h2, 1 mole of o2 and 1 mole of f2
(a)which has the largest number of molecul...
(a)which has the largest number of molecul...
Questions

Mathematics, 30.07.2020 14:01


History, 30.07.2020 14:01






Chemistry, 30.07.2020 14:01


Mathematics, 30.07.2020 14:01



Mathematics, 30.07.2020 14:01

Mathematics, 30.07.2020 14:01

Social Studies, 30.07.2020 14:01



Social Studies, 30.07.2020 14:01

Mathematics, 30.07.2020 14:01

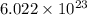 atoms or molecules, that is, Avogadro's number of atoms.
atoms or molecules, that is, Avogadro's number of atoms.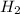 =
= 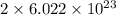 molecules
molecules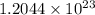 molecules
molecules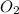 =
= 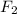 =
=