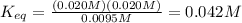Consider the reaction below.
at 500 k, the reaction is at equilibrium with the following conce...

Chemistry, 01.09.2019 17:20 vanleervanleer
Consider the reaction below.
at 500 k, the reaction is at equilibrium with the following concentrations.
[pci5]= 0.0095 m
[pci3] = 0.020
[ci2] = 0.020 m
what is the equilibrium constant for the given reaction?
0.042
0.42
2.4
24
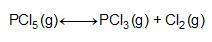

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Drive down any three characteristic of modern periodic table
Answers: 1

Chemistry, 22.06.2019 08:30
Which of the following would be an accurate picture of the earth during the summer time of the northern hemisphere?
Answers: 1

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1

Chemistry, 22.06.2019 17:00
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1
You know the right answer?
Questions

Mathematics, 18.11.2020 05:50

Mathematics, 18.11.2020 05:50



Mathematics, 18.11.2020 05:50





Mathematics, 18.11.2020 05:50


Mathematics, 18.11.2020 05:50




Biology, 18.11.2020 05:50



Mathematics, 18.11.2020 05:50

![K_{eq}=\frac{[PCl_3]^1[Cl_2]^1}{[PCl_5]^1}](/tpl/images/0218/2590/d25fc.png)