The following data was measured for the reaction above at a constant temperature.
experiment i...

Chemistry, 31.08.2019 05:30 4300224102
The following data was measured for the reaction above at a constant temperature.
experiment initial [a] mol l‐1 initial [b] mol l‐1 initial rate of formation of c (mol l‐1 s‐1)
1 0.10 0.10 1.12 x 10‐3
2 0.10 0.20 2.24 x 10‐3
3 0.20 0.10 4.48 x 10‐3
what is the order of the reaction with respect to a?
what is the order of the reaction with respect to b?
what is the rate law for this reaction?
calculate the rate constant.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which of the following would have less momentum than a 52 kg cheetah running at 10 m/s?
Answers: 2

Chemistry, 22.06.2019 11:20
Which of the following contributes to the structural rigidity of cellulose? adjacent glucose polymers are stabilized by hydrogen bonding. glucose residues are joined by (α1→4) linkages. cellulose is a highly branched molecule. the conformation of the glucose polymer is a coiled structure.
Answers: 2

Chemistry, 22.06.2019 14:30
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1

Chemistry, 22.06.2019 22:30
Calculate the concentration of all species in a 0.165 m solution of h2co3.
Answers: 1
You know the right answer?
Questions










Mathematics, 19.03.2020 01:31






Biology, 19.03.2020 01:31


Computers and Technology, 19.03.2020 01:32

Spanish, 19.03.2020 01:32

![V=k[A]^{2} [B]](/tpl/images/0214/1245/29401.png)
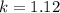
![\frac{[A]_{3}}{[A]_1{}} =2](/tpl/images/0214/1245/57061.png)
![\frac{[V]_{3}}{[V]_1{}} =4](/tpl/images/0214/1245/908c3.png)
![[A]^{2}](/tpl/images/0214/1245/66fd2.png)
![\frac{[B]_{2}}{[B]_1{}} =2](/tpl/images/0214/1245/5cc2d.png)
![\frac{[V]_{2}}{[V]_1{}} =2](/tpl/images/0214/1245/0fa6f.png)
![[B]}](/tpl/images/0214/1245/fe23b.png)
![V=k[A]^{2}[B]](/tpl/images/0214/1245/202fc.png)
![k=\frac{V}{[A]^{2}[B]}](/tpl/images/0214/1245/4243e.png)


