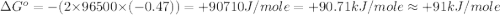Chemistry, 31.08.2019 05:30 elizabethcswind5062
Use the provided reduction potentials to calculate argº for the following balanced redox reaction: pb2+(aq) + cu(s) → pb(s) + cu2+(aq) e°(pb2+/pb) = -0.13 v and e°(cu2+/cu) = +0.34 v -41 kj mol-1 +91 kj mol-1 -21 kj mol-1 -0.47 kj mol-1 +46 kj mol-1

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 12:30
Which of the following can be used to measure electricity
Answers: 1

Chemistry, 22.06.2019 02:20
Compared with the freezing-point depression of a 0.01 m c6h12o6 solution, the freezing-point depression of a 0.01 m nacl solution is
Answers: 1

Chemistry, 22.06.2019 02:50
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2

Chemistry, 22.06.2019 03:00
Compare the valence electron configuration of the nobles gas elements seen here. what statement is correct?
Answers: 2
You know the right answer?
Use the provided reduction potentials to calculate argº for the following balanced redox reaction:...
Questions

Mathematics, 25.05.2021 21:20

Social Studies, 25.05.2021 21:20

Mathematics, 25.05.2021 21:20

History, 25.05.2021 21:20



English, 25.05.2021 21:20

Social Studies, 25.05.2021 21:20

Mathematics, 25.05.2021 21:20

Mathematics, 25.05.2021 21:20



Mathematics, 25.05.2021 21:20



History, 25.05.2021 21:20


Spanish, 25.05.2021 21:20

Mathematics, 25.05.2021 21:20

Chemistry, 25.05.2021 21:20

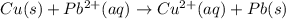
![E^0_{[Pb^{2+}/Pb]}=-0.13V](/tpl/images/0214/1462/82211.png)
![E^0_{[Cu^{2+}/Cu]}=+0.34V](/tpl/images/0214/1462/ecde5.png)
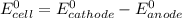
![E^0_{cell}=E^0_{[Pb^{2+}/Pb]}-E^0_{[Cu^{2+}/Cu]}](/tpl/images/0214/1462/6ec7d.png)
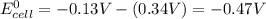
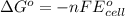
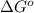 = standard Gibbs free energy = ?
= standard Gibbs free energy = ?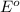 = standard e.m.f of cell = -0.47 V
= standard e.m.f of cell = -0.47 V