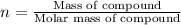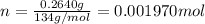Chemistry, 31.08.2019 02:30 pearpeaerrr1993
3.0.2640 g of sodium oxalate is dissolved in a flask and requires 30.74 ml of potassium permanganate to titrate it to turn pink end point. the equation for this reaction is: 5na, c,o4+ 2kmno,+ 8h, so 2mnsog+ k, so,+ 5 na, so,+ 10co2+ 8h2o a) how many moles of sodium oxalate are present in the flask?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Temperature and kinetic energy are proportional. a) adirectly b) directly c) indirectly
Answers: 2

Chemistry, 22.06.2019 02:30
Select each correct answer. more than one answer may be correct. which of the following is a characteristic of unicellular organisms? they can possess tissues and organs. all of their functions are performed by a single cell. they are usually microscopic. each of their cells is specialized to perform a specific function.
Answers: 1

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

You know the right answer?
3.0.2640 g of sodium oxalate is dissolved in a flask and requires 30.74 ml of potassium permanganate...
Questions

Mathematics, 31.05.2020 20:58

Mathematics, 31.05.2020 20:58

Mathematics, 31.05.2020 20:58

Mathematics, 31.05.2020 20:58

Mathematics, 31.05.2020 20:58

Mathematics, 31.05.2020 20:58


Mathematics, 31.05.2020 20:58

Mathematics, 31.05.2020 20:58

Mathematics, 31.05.2020 20:58




Geography, 31.05.2020 20:58



History, 31.05.2020 20:58


Mathematics, 31.05.2020 20:59

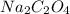 = 134 g/mol
= 134 g/mol