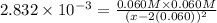Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2

Chemistry, 22.06.2019 10:30
Skills of homo sapiens were found an excavation. the skulls were preserved because the bodies were frozen. so, these fossils are (blank) fossils.the image shows the evolution of skulls beginning 2 to 3 million years ago. based on the image, modern human skulls(blank) ape skulls.
Answers: 1

Chemistry, 22.06.2019 11:50
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3

Chemistry, 22.06.2019 21:30
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3
You know the right answer?
Write the equilibrium reactions on a scratch paper, calculate k from ksp and kf and determine the co...
Questions

History, 19.01.2021 19:30

English, 19.01.2021 19:30

Biology, 19.01.2021 19:30

History, 19.01.2021 19:30

Health, 19.01.2021 19:30

English, 19.01.2021 19:30

Mathematics, 19.01.2021 19:30

Biology, 19.01.2021 19:30



English, 19.01.2021 19:30




Medicine, 19.01.2021 19:30


Health, 19.01.2021 19:30


English, 19.01.2021 19:30

Mathematics, 19.01.2021 19:30

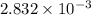 .
.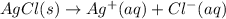
![K_{sp}=1.77\times 10^{-10}=[Ag^+][Cl^-]](/tpl/images/0213/7276/ad131.png) ..(1)
..(1)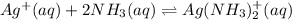
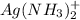 :
:![K_f=1.6\times 10^7=\frac{[Ag(NH_3)_2^{+}]}{[Ag^+][NH_3]^2}](/tpl/images/0213/7276/1553b.png) ..(2)
..(2)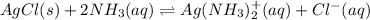
![K=\frac{[Ag(NH_3)_2^{+}][Cl^-]}{[AgCl][NH_3]^2}](/tpl/images/0213/7276/55892.png)
![K=\frac{[Ag(NH_3)_2^{+}][Cl^-]}{[1][NH_3]^2}\times \frac{[Ag^+]}{[Ag^+]}](/tpl/images/0213/7276/23d55.png)
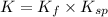 (from 1 and 2)
(from 1 and 2)
![[Ag(NH_3)_2^{+}]](/tpl/images/0213/7276/05dbb.png) = 0.060 M
= 0.060 M![K=\frac{[Ag(NH_3)_2^{+}][Cl^-]}{[1][NH_3]^2}](/tpl/images/0213/7276/bb0d2.png)