

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:00
This image is an example of a(n) a) atom. b) compound. c) mixture. d) molecule.
Answers: 1

Chemistry, 22.06.2019 10:50
How many liters of oxygen gas, at standard temperature and pressure, will react with 35.8 grams of iron metal? 4 fe (s) + 3 o₂ (g) → 2 fe₂o₃ (s)
Answers: 2

Chemistry, 22.06.2019 11:30
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2

Chemistry, 22.06.2019 13:50
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2
You know the right answer?
Which hybrid orbitals are used to form the sigma bonds between the carbons in acetylene? (blb ch. 9...
Questions




English, 06.11.2019 06:31


History, 06.11.2019 06:31


Social Studies, 06.11.2019 06:31


History, 06.11.2019 06:31



Mathematics, 06.11.2019 06:31


Mathematics, 06.11.2019 06:31

Mathematics, 06.11.2019 06:31



English, 06.11.2019 06:31

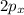 orbital and two sp hybrid orbitals are formed which are oriented at the angle of 180° . One is bonded to hydrogen and another to the carbon.
orbital and two sp hybrid orbitals are formed which are oriented at the angle of 180° . One is bonded to hydrogen and another to the carbon. 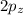 and
and 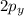 orbitals remain non-hybridized and thus are oriented perpendicularly along z and y axes respectively.
orbitals remain non-hybridized and thus are oriented perpendicularly along z and y axes respectively.

