Which of the following metal cations is the strongest oxidizing agent?
a) cu2+; eocu2+/cu =...

Chemistry, 30.08.2019 23:30 officialgraciela67
Which of the following metal cations is the strongest oxidizing agent?
a) cu2+; eocu2+/cu = + 0.34 v
b) al3+; eoal3+/al = -1.66 v
c) sn2+; eosn2+/sn = -0.14 v
d) ag+; eoag+/ag = + 0.80 v
e) mn2+; eomn2+/mn = -1.18 v

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:40
Achemistry student weighs out of phosphoric acid , a triprotic acid, into a volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with solution. calculate the volume of solution the student will need to add to reach the final equivalence point. round your answer to significant digits.
Answers: 3

Chemistry, 22.06.2019 09:30
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1

Chemistry, 22.06.2019 14:30
The valence of aluminum is +3, and the valence of the chlorine is -1. the formula fir the aluminum chloride is correctly written as
Answers: 2

Chemistry, 22.06.2019 18:00
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3
You know the right answer?
Questions

Biology, 06.04.2021 23:40

Mathematics, 06.04.2021 23:40


Mathematics, 06.04.2021 23:40



Mathematics, 06.04.2021 23:40


English, 06.04.2021 23:40


Mathematics, 06.04.2021 23:40

Mathematics, 06.04.2021 23:40



History, 06.04.2021 23:40


Mathematics, 06.04.2021 23:40

Health, 06.04.2021 23:40


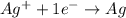
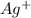 is the oxidizing agent.
is the oxidizing agent.

