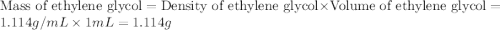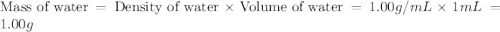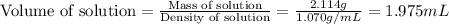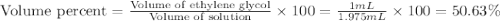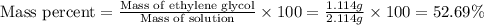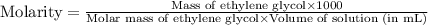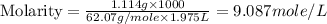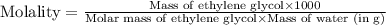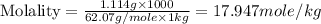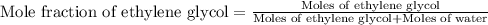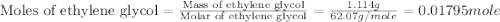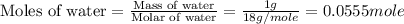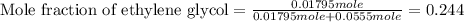An automobile antifreeze mixture is made by mixing equal volumes of ethylene glycol (d = 1.114 g/ml; m = 62.07 g/mol) and water (d = 1.00 g/ml) at 20°c. the density of the mixture is 1.070 g/ml. express the concentration of ethylene glycol as (a) volume percent 50 % v/v (b) mass percent 52.7 % w/w (c) molarity m (d) molality m (e) mole fraction

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
The agent of mechanical weathering in which rock is worn away by the grinding action of other rock particles is call
Answers: 1

Chemistry, 22.06.2019 10:30
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2

Chemistry, 23.06.2019 06:00
What physical property of gold makes panning a useful way to get gold from streams?
Answers: 2

Chemistry, 23.06.2019 09:00
Need ! assume that the variables x and y are directly related. if k = 8, what is the value for each of the following points? be sure and record your data to be used in the following problem. x y k 0.
Answers: 2
You know the right answer?
An automobile antifreeze mixture is made by mixing equal volumes of ethylene glycol (d = 1.114 g/ml;...
Questions


English, 01.03.2021 01:50



Mathematics, 01.03.2021 01:50



Biology, 01.03.2021 01:50

History, 01.03.2021 01:50



History, 01.03.2021 01:50

Mathematics, 01.03.2021 01:50



History, 01.03.2021 01:50


Mathematics, 01.03.2021 01:50

History, 01.03.2021 01:50