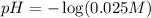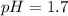Chemistry, 30.08.2019 20:10 azalialujan4634
A100.0 ml sample of 0.10 m ca(oh)2 is titrated with 0.10 m hbr. determine the ph of the solution after the addition of 300.0 ml hbr. a) 2.62 b)2.00 c) 1.7 d) 12.52

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Apiece of metal with a length of 1.42 cm was measured using four different instruments. which of the following measurements is the most accurate?
Answers: 3

Chemistry, 22.06.2019 19:20
For a research project, a student decided to test the effect of the lead(ii) ion (pb2+) on the ability of salmon eggs to hatch. this ion was obtainable from the water‐soluble salt, lead(ii) nitrate, which the student decided to make by the following reaction. pbo(s) + 2 hno3(aq) → pb(no3)2(aq) + h2o losses of product for various reasons were expected, and a yield of 86.0% was expected. in order to have 5.00 g of product at this yield, how many grams of pbo should be reacted? (assume that sufficient nitric acid, hno3, would be used.)
Answers: 1

Chemistry, 22.06.2019 20:00
State one important difference between a physical change and a chemical change?
Answers: 1

Chemistry, 23.06.2019 02:00
The bohr model of the atom explained why emission spectra are discrete. it could also be used to explain the photoelectric effect. which is a correct explanation of the photoelectric effect according to the model?
Answers: 3
You know the right answer?
A100.0 ml sample of 0.10 m ca(oh)2 is titrated with 0.10 m hbr. determine the ph of the solution aft...
Questions

Mathematics, 11.03.2021 04:40

Social Studies, 11.03.2021 04:40

Mathematics, 11.03.2021 04:40


Mathematics, 11.03.2021 04:40

Geography, 11.03.2021 04:40

Mathematics, 11.03.2021 04:40


Advanced Placement (AP), 11.03.2021 04:40


Mathematics, 11.03.2021 04:40

History, 11.03.2021 04:40

English, 11.03.2021 04:40


Law, 11.03.2021 04:40

Computers and Technology, 11.03.2021 04:40


English, 11.03.2021 04:40

Arts, 11.03.2021 04:40

Mathematics, 11.03.2021 04:40

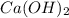 and
and 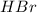 .
.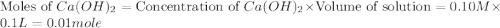
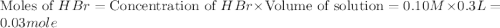
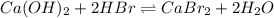
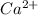 ion and 0.02 mole of
ion and 0.02 mole of 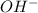 ion
ion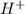 ion and 0.03 mole of
ion and 0.03 mole of 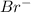 ion
ion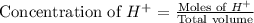
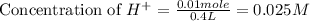
![pH=-\log [H^+]](/tpl/images/0212/8760/37e81.png)